A Compendium Of Battery Information
Downloadable article as a PDF by clicking here.
First, a little history –
Although Alessandro Volta in Italy is usually credited with being the inventor of the modern battery (Silver-Zinc), ancient cells have been discovered in Sumerian ruins, origin around 250 BC.. or maybe 400 AD – there is some dispute over the exact age. The first evidence of batteries comes from archaeological digs in Baghdad, Iraq. his first “battery” was dated to around 250 (?) B.C. and was used in simple operations to electroplate objects with a thin layer of metal, much like the process used now to plate inexpensive gold and silver jewelry [This is also debated, the current put out by these batteries is very small, so they may have been no more than a witch doctors props to amaze patients with electrical tingles].
The jars were found in Khujut Rabu just outside Baghdad and are composed of a clay jar with a stopper made of asphalt. Sticking through the asphalt is an iron rod surrounded by a copper cylinder. When filled with vinegar or even grape juice – or any other electrolytic solution – the jar produces about 1.1 volts. The actual term “battery” was coined by Benjamin Franklin to describe an array of static charged glass plates (sort of a primitive capacitor). He used the term battery, as that term was common at the time for a group of guns or artillery.
Batteries were re-discovered much later, in 1800 in Italy by a man named Alessandro Volta after which the unit of electrical potential was named, the volt. His original battery consisted of discs of Zinc and Copper in a large glass tube separated by cardboard. The French inventor, Gaston Plante developed the first practical Lead-Acid rechargeable storage battery in 1859. In 1881 Faure discovered a way to make a Lead “paste”, which greatly improved the capacity of batteries. This is basically the same method and chemistry used in most batteries today.
Most early batteries were used for telegraphs, and research and development was slow until the automobile came along. Some of the first cars were electric, and relied on these rather primitive batteries to get around. With the suddenly increased demand for rechargeable batteries, the modern battery started to take shape. One of the first commercial car batteries was made with an Oak wood case with Red Cedar plate separators. A variety of other woods were used, but Cedar was most common. The cases were dipped in asphalt, assembled, and then the top was glued, tied, screwed, or latched on. Up until around 1916 it was quite common to have these batteries repaired, so they had to be easy to take apart. A few “luxury” batteries also used glass cases, and a variety of what we would consider strange materials were tried by some manufacturers – including kiln fired clay and heavily waxed leather. Needless to say, reliability was not a strong point, and neither was safety.
Rubber plate separators were introduced by Willard Battery Company in 1915. Shortly after, Willard and several other companies started using hard rubber cases. These were common in car batteries until the invention of easily molded plastics, like Bakelite, prior to WW2. The first gelled VRLA battery was made in 1958, and Polypropylene cases became common in the mid to late 60’s. In 1980 the first AGM (absorbed glass mat) battery was invented, mainly for military use. Up until the early 90’s AGM batteries were very uncommon outside the military, but then around 1991 Concorde battery began selling its military line of batteries to the civilian market. Accu Oerlikin and several other manufacturers followed soon after.
What is a battery?
There are two classes of batteries, primary and secondary. No idea why they are called that, but primary cannot be recharged, secondary can be. A battery, in concept, can be any device that stores energy for later use. A rock, pushed to the top of a hill, can be considered a kind of battery, since the energy used to push it up the hill
(chemical energy, from muscles or combustion engines) is converted and stored as potential kinetic energy at the top of the hill. Later, that energy is released as kinetic and thermal energy when the rock rolls down the hill. Common use of the word, “battery,” however, is usually limited to an electrochemical device that converts chemical energy into electricity, by use of a galvanic cell. A galvanic cell is a fairly simple device consisting of two electrodes (an anode and a cathode) and an
electrolyte solution. Batteries consist of one or more galvanic cells.
A battery is an electrical storage device. Batteries do not make electricity, they store it, just as a water tank stores water for future use. As chemicals in the battery change, electrical energy is stored or released. In rechargeable batteries this process can be repeated many times. Batteries are not 100% efficient – some energy is lost as heat and chemical reactions when charging and discharging. If you use 1000 watts from a battery, it might take 1200 watts or more to fully recharge it. Slower charging and discharging rates are more efficient. A battery rated at 180 amp-hours over 6 hours might be rated at 220 AH at the 20-hour rate, and 260 AH at the 48-hour rate. Typical efficiency in a lead-acid battery is 85-95%, in alkaline nickel-iron and nickel cadmium batteries, it is 65% or less.
It is important to note that most of the batteries commonly used in deep cycle applications are lead-acid. This includes the standard flooded (wet) batteries, gelled, and AGM. They all use the same chemistry, although the actual construction of the plates etc can vary considerably. Nickel cadmiums, nickel-iron, and other types are found in some systems, but are not common due to their expense, availability, – they are generally only used in very unusual or demanding applications, such as aircraft, transit vehicles, ships, etc.
Major Battery Types
Batteries are divided in two ways, by application (what they are used for) and construction (how they are built). The major applications are automotive, marine, and deep-cycle. Deep-cycle includes solar electric, backup power, RV and boat “house” batteries. The major construction types are flooded (wet), gelled, and AGM (Absorbed Glass Mat). AGM batteries are also sometimes called “starved electrolyte” or “dry”, because the fiberglass mat is only 95% saturated with sulfuric acid and there is no excess liquid
Flooded may be standard, with removable caps, or the so-called “maintenance free” (that means they are designed to die one week after the warranty runs out). All gelled are sealed and a few are “valve regulated”, which means that a tiny valve keeps a slight positive pressure. Nearly all AGM batteries are sealed valve regulated (commonly referred to as “VRLA” – Valve Regulated Lead-acid). Most valve regulated are under some pressure – 1 to 4 psi at sea level.
Lifespan of Batteries
The lifespan of a battery will vary considerably with how it is used, how it is maintained and charged, temperature, and other factors. In extreme cases, it can vary to extremes – good batteries can be killed in less than a year by severe overcharging, but a large set of surplus telephone batteries that sees only occasional (5-10 times per year) heavy service can be over 25 years old. Gelled cells will be destroyed in one day when overcharged with a large automotive charger. Golf cart batteries have been destroyed without ever being used in less than a year because they were left sitting in a hot garage without being charged. Even the so-called “dry charged” (where you add acid when you need them) have a shelf life of at most 18 months, as they are not totally dry (actually, a few are, but hard to find, the vast majority are shipped with
damp plates).
These are some general (minimum – maximum) typical expectations for batteries if used in deep cycle service:
- Starting: 3-12 months
- Marine: 1-6 years
- Golf cart: 2-6 years
- AGM deep cycle: 4-7 years (this can vary considerably – the large 2 volt cells can last for 20+)
- Gelled deep cycle: 2-5 years
- Deep cycle (L-16 type etc): 4-8 years
- Rolls-Surrette premium deep cycle: 7-15 years
- Industrial deep cycle (Crown and Rolls 4KS series): 10-20+ years
- Telephone (used) (float): 1-20 years. These are usually special purpose “float service”, but often appear on the surplus market as “deep cycle”. They can vary considerably, depending on age, usage, care, and type.
- Nickel- iron (alkaline): 5-35 years
- Nickel-cadmium (alkaline): 1-20 years
Starting, Marine, and Deep-Cycle Batteries
- Starting (sometimes called SLI, for starting, lighting, ignition) batteries are commonly used to start and run engines. Engine starters need a very large starting current for a very short time. Starting batteries have a large number of thin plates for maximum surface area. The plates are composed of a Lead “sponge”, similar in appearance to a very fine foam sponge. This gives a very large surface area, but if deep cycled, this sponge will quickly be consumed and fall to the bottom of the cells. Automotive batteries will generally fail after 30-150 deep cycles if deep cycled, while they may last for thousands of cycles in normal starting use (2-5% discharge).
- Deep cycle batteries are designed to be discharged down as much as 80% time after time, and have much thicker plates. The major difference between a true deep cycle battery and others is that the plates are SOLID Lead plates – not sponge. Unfortunately, it is often impossible to tell what you are really buying in some of the discount stores or places that specialize in automotive batteries. The popular golf cart battery is generally a “semi” deep cycle – better than any starting battery, better than most marine, but not as good as a true deep cycle solid Lead plate, such the L-16 or industrial type. However, because the golf cart (T-105, US-2200, GC-4 etc)
batteries are so common, they are usually quite economical for small to medium systems. - Many (most?) Marine batteries are usually actually a “hybrid”, and fall between the starting and deep-cycle batteries, while a few (Rolls-Surrette and Concorde, for example) are true deep cycle. In the hybrid, the plates may be composed of Lead sponge, but it is coarser and heavier than that used in starting batteries. It is often hard to tell what you are getting in a “marine” battery, but most are a hybrid. “Hybrid” types should not be discharged more than 50%. Starting batteries are usually rated at “CCA”, or cold cranking amps, or “MCA”, Marine cranking amps – the same as “CA”. Any battery with the capacity shown in CA or MCA may not be a true deepcycle battery. It is sometimes hard to tell, as the terms marine and deep cycle are sometimes overused. CA and MCA ratings are at 32 degrees F, while CCA is at zero degree F. Unfortunately, the only positive way to tell with some batteries is to buy one and cut it open – not much of an option.
Battery Construction Materials
Nearly all large rechargeable batteries in common use are Lead-acid type. (There are some NiCads in use, but for most purposes the very high initial expense, and the high expense of disposal, does not justify them). The acid is typically 30% Sulfuric acid and 70% water at full charge. NiFe (Nickel-Iron) batteries are also available – these have a very long life, but rather poor efficiency (60-70%) and the voltages are different, making it more difficult to match up with standard 12v/24/48v systems and inverters. The biggest problem with NiFe batteries is that you may have to put in 100 watts to get 70 watts of charge – they are much less efficient than Lead-acid. What you save on batteries you will have to make up for by buying a larger solar panel system. NiCads are also inefficient – typically around 65% – and very expensive. However, NiCads can be frozen without damage, so are sometimes used in areas where the temperatures may fall below -50 degrees F. Most AGM batteries will also survive freezing with no problems, even though the output when frozen will be little or nothing.
Industrial deep cycle batteries
Sometimes called “fork lift”, “traction” or “stationary” batteries, are used where power is needed over a longer period of time, and are designed to be “deep cycled”, or discharged down as low as 20% of full charge (80% DOD, or Depth of Discharge). These are often called traction batteries because of their widespread use in forklifts, golf carts, and floor sweepers (from which we get the “GC” and “FS” series of battery sizes). Deep cycle batteries have much thicker plates than automotive batteries.
Plate Thickness
Plate thickness (of the Positive plate) matters because of a factor called “positive grid corrosion“. This ranks among the top 3 reasons for battery failure. The positive (+) plate is what gets eaten away gradually over time, so eventually there is nothing left – it all falls to the bottom as sediment. Thicker plates are directly related to longer life, so other things being equal, the battery with the thickest plates will last the longest.
Automotive batteries typically have plates about .040″ (40/1000″) thick, while forklift batteries may have plates more than 1/4″ (.265″ for example in the Rolls-Surrette) thick – almost 7 times as thick as auto batteries. The typical golf cart will have plates that are around .07 to .11″ thick. The Concorde AGM’s are .115″, The Rolls-Surrette L-16 type (CH460) is .150″, and the US Battery and Trojan L-16 types are .090”.
Most industrial deep-cycle batteries use Lead-Antimony plates rather than the Lead-Calcium used in AGM or gelled deep-cycle batteries. The Antimony increases plate life and strength, but increases gassing and water loss. This is why most industrial batteries have to be checked often for water level if you do not have Hydrocaps. The self discharge of batteries with Lead-Antimony plates can be high – as much as 1% per day on an older battery. A new AGM typically self-discharges at about 1-2% per month, while an old one may be as much as 2% per week.
Sealed batteries
Sealed batteries are made with vents that (usually) cannot be removed. The so-called Maintenance Free batteries are also sealed, but are not usually leak proof. Sealed batteries are not totally sealed, as they must allow gas to vent during charging. If overcharged too many times, some of these batteries can lose enough water that they will die before their time. Most smaller deep cycle batteries (including AGM) use Lead-Calcium plates for increased life, while most industrial and forklift batteries use Lead-Antimony for greater plate strength.
A few industrial batteries have special caps that convert the Hydrogen and Oxygen back into water, reducing water loss by up to 95%. The popular “HydroCaps” that sold for flooded batteries do the same job for conventional (“wet”), golf cart, and forklift batteries. Lead-Antimony batteries have a much higher self-discharge rate (2-10% per week) than Lead or Lead-Calcium (1-5% per month), but the Antimony improves the mechanical strength of the plates, which is an important factor in electric vehicles. They are generally used where they are under constant or very frequent charge/discharge cycles, such as fork lifts and floor sweepers. The Antimony increases plate life at the expense of higher self discharge. If left for long periods unused, these should be trickle charged to avoid damage from sulfation – but this applies to ANY battery.
As in all things, there are trade offs. The Lead-Antimony types have a very long lifespan, but higher self discharge rates.
Battery Size Codes
Batteries come in all different sizes. Many have “group” sizes, which is based upon the physical size and terminal placement. It is NOT a measure of battery capacity. Typical BCI codes are group U1, 24, 27, and 31. Industrial batteries are usually designated by a part number such as “FS” for floor sweeper, or “GC” for golf cart. Many batteries follow no particular code, and are just manufacturers part numbers. Other standard size codes are 4D & 8D, large industrial batteries, commonly used in solar electric systems.
Some common battery size codes used are: (ratings are approximate)
- U1 34 to 40 Amp hours 12 volts
- Group 24 70-85 Amp hours 12 volts
- Group 27 85-105 Amp hours 12 volts
- Group 31 95-125 Amp hours 12 volts
- 4-D 180-215 Amp hours 12 volts
- 8-D 225-255 Amp hours 12 volts
- Golf cart & T-105 180 to 220 Amp hours 6 volts
- L-16 340 to 415 Amp hours 6 volts
Gelled electrolyte
Gelled batteries, or “Gel Cells” contain acid that has been “gelled” by the addition of Silica Gel, turning the acid into a semi-solid mass that looks like gooey Jell-O. The advantage of these batteries is that it is impossible to spill acid even if they are broken. However, there are several disadvantages. One is that they must be charged at a slower rate (C/20 maximum) to prevent excess gas from damaging the cells and causing bubbles or pockets in the electrolyte. They cannot be fast charged on conventional automotive charger or they may be permanently damaged. This is not usually a problem with solar electric systems, but if an auxiliary generator or inverter bulk charger is used, current must be limited to the manufacturers’ specifications. Most better inverters commonly used in solar electric systems can be set to limit charging current to the batteries.
Some other disadvantages of gel cells is that they must be charged at a lower voltage (2/10th’s less) than flooded or AGM batteries. If overcharged, voids can develop in the gel which will never heal, causing a loss in battery capacity. In hot climates, water loss can be enough over 2-4 years to cause premature battery death. It is for this and other reasons that we no longer sell any of the gelled cells except for replacement use. The newer AGM (absorbed glass mat) batteries have all the advantages and then some) of gelled, with none of the disadvantages.
AGM, or Absorbed Glass Mat Batteries
A newer type of sealed battery uses “Absorbed Glass Mats”, or AGM between the plates. This is a very fine fiber Boron-Silicate glass mat. These type of batteries have all the advantages of gelled, but can take much more abuse. We sell the Concorde (and Lifeline, made by Concorde) AGM batteries. These are also called “starved electrolyte”, as the mat is about 95% saturated rather than fully soaked. That also means that they will not leak acid even if broken. AGM batteries have several advantages over both gelled and flooded, at about the same cost as gelled: Since all the electrolyte (acid) is contained in the glass mats, they cannot spill, even if broken. This also means that since they are non-hazardous, the shipping costs are lower. In addition, since there is no liquid to freeze and expand, they are practically immune from freezing damage.
Nearly all AGM batteries are “recombinant” – what that means is that the Oxygen and Hydrogen recombine INSIDE the battery. These use gas phase transfer of oxygen to the negative plates to recombine them back into water while charging and prevent the loss of water through electrolysis. The recombining is typically 99+% efficient, so almost no water is lost. The charging voltages are the same as for any standard battery – no need for any special adjustments or problems with incompatible chargers or charge controls. And, since the internal resistance is extremely low, there is almost no heating of the battery even under heavy charge and discharge currents. The Concorde (and most AGM) batteries have no charge or discharge current limits.
AGM’s have a very low self-discharge – from 1% to 3% per month is usual. This means that they can sit in storage for much longer periods without charging than standard batteries. The Concorde batteries can be almost fully recharged (95% or better) even after 30 days of being totally discharged. AGM’s do not have any liquid to spill, and even under severe overcharge conditions hydrogen emission is far below the 4% max specified for aircraft and enclosed spaces. The plates in AGM’s are tightly packed and rigidly mounted, and will withstand shock and vibration better than any standard battery.
Even with all the advantages listed above, there is still a place for the standard flooded deep cycle battery. AGM’s will cost about twice as much as good flooded
batteries of the same capacity. In many installations, where the batteries are set in an area where you don’t have to worry about fumes or leakage, a standard or industrial deep cycle is a better economic choice. AGM batteries main advantages are no maintenance, completely sealed against fumes, Hydrogen, or leakage, non-spilling even if they are broken, and can survive most freezes. Not everyone needs these features.
Temperature Effects on Batteries
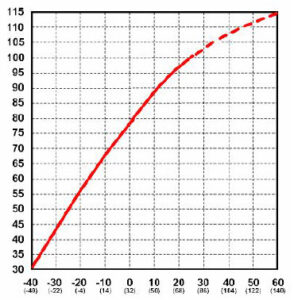
Battery capacity (how many amp-hours it can hold) is reduced as temperature goes down, and increased as temperature goes up. This is why your car battery dies on a cold winter morning, even though it worked fine the previous afternoon. If your batteries spend part of the year shivering in the cold, the reduced capacity has to be taken into ccount when sizing the system batteries. The standard rating for batteries is at room temperature – 25 degrees C (about 77 F). At approximately -22 degrees F (-27 C), battery AH capacity drops to 50%. At freezing, capacity is reduced by 20%. Capacity is increased at higher temperatures – at 122 degrees F, battery capacity would be about 12% higher. Battery charging voltage also changes with temperature. It will vary from about 2.74 volts per cell (16.4 volts) at -40 C to 2.3 volts per cell (13.8 volts) at 50 C. This is why you should have temperature compensation on your charger or charge control if your batteries are outside and/or subject to wide temperature variations. Some charge controls have temperature compensation built in (such as Morningstar) – this works fine if the controller is subject to the same temperatures as the batteries. However, if your batteries are outside, and the controller is inside, it does not work that well. Adding another complication is that large battery banks make up a large thermal mass.
Thermal mass means that because they have so much mass, they will change internal temperature much slower than the surrounding air temperature. A large insulated battery bank may vary as little as 10 degrees over 24 hours internally, even though the air temperature varies from 20 to 70 degrees. For this reason, external (add-on) temperature sensors should be attached to one of the POSITIVE plate terminals, and bundled up a little with some type of insulation on the terminal. The sensor will then read very close to the actual internal battery temperature.
Even though battery capacity at high temperatures is higher, battery life is shortened. Battery capacity is reduced by 50% at -22 degrees F – but battery LIFE increases by about 60%. Battery life is reduced at higher temperatures – for every 15 degrees F over 77, battery life is cut in half. This holds true for ANY type of Lead-acid battery, whether sealed, gelled, AGM, industrial or whatever. This is actually not as bad as it seems, as the battery will tend to average out the good and bad times.
One last note on temperatures – in a few places that have extremely cold or hot conditions, batteries may be sold locally that are NOT standard electrolyte (acid) strengths. The electrolyte may be stronger (for cold) or weaker (for very hot) climates. In such cases, the specific gravity and the voltages may vary from what we show for flooded batteries.
Cycles vs Life
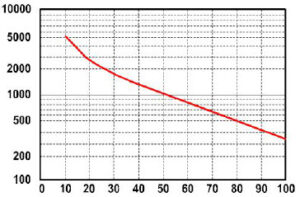
A battery “cycle” is one complete discharge and recharge cycle. It is usually considered to be discharging from 100% to 20%, and then back to 100%. However, there are often ratings for other depth of discharge cycles, the most common ones are 10%, 20%, and 50%. You have to be careful when looking at ratings that list how many cycles a battery is rated for unless it also states how far down it is being discharged. For example, one of the widely advertised telephone type (float service) batteries have been advertised as having a 20-year life. If you look at the fine print, it has that rating only at 5% DOD – it is much less when used in an application where they are cycled deeper on a regular basis. Those same batteries are rated at less than 5 years if cycled to 50%. For example, most golf cart batteries are rated for about 550 cycles to 50% discharge – which equates to about 2 years.
Battery life is directly related to how deep the battery is cycled each time. If a battery is discharged to 50% every day, it will last about twice as long as if it is cycled to 80% DOD. If cycled only 10% DOD, it will last about 5 times as long as one cycled to 50%. Obviously, there are some practical limitations on this – you don’t usually want to have a 5 ton pile of batteries sitting there just to reduce the DOD. The most practical number to use is 50% DOD on a regular basis. This does NOT mean you cannot go to 80% once in a while. It’s just that when designing a system when you have some idea of the loads, you should figure on an average DOD of around 50% for the best storage vs cost factor. Also, there is an upper limit – a battery that is continually cycled 5% or less will usually not last as long as one cycled down 10%. This happens because at very shallow cycles, the Lead Dioxide tends to build up in clumps on the the positive plates rather in an even film. The graph above shows how lifespan is affected by depth of discharge. The chart is for a Concorde Lifeline battery, but all lead-acid batteries will be similar in the shape of the curve, although the number of
cycles will vary.
Battery Voltages
All Lead-acid batteries supply about 2.14 volts per cell (12.6 to 12.8 for a 12 volt battery) when fully charged. Batteries that are stored for long periods will eventually lose all their charge. This “leakage” or self discharge varies considerably with battery type, age, & temperature. It can range from about 1% to 15% per month. Generally, new AGM batteries have the lowest, and old industrial (lead-antimony plates) are the highest. In systems that are continually connected to some type charging source, whether it is solar, wind, or an AC powered charger this is seldom a problem. However, one of the biggest killers of batteries is sitting stored in a partly discharged state for a few months. A “float” charge should be maintained on the batteries even if they are not used (or, especially if they are not used). Even most “dry charged” batteries (those sold without electrolyte so they can be shipped more easily, with acid added later) will deteriorate over time. Max storage life on those is about 2-3 years.
Batteries self-discharge faster at higher temperatures. Lifespan can also be seriously reduced at higher temperatures – most manufacturers state this as a 50% loss in life for every 15 degrees F over a 77 degree cell temperature. Lifespan is increased at the same rate if below 77 degrees, but capacity is reduced. This tends to even out in most systems – they will spend part of their life at higher temperatures, and part at lower.
Myth: The old myth about not storing batteries on concrete floors is just that – a myth. This old story has been around for 100 years, and originated back when battery cases were made up of wood and asphalt. The acid would leak from them, and form a slow-discharging circuit through the now acid-soaked and conductive floor.
State of Charge
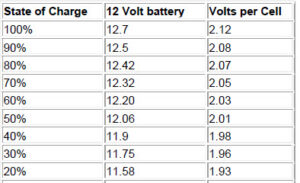
State of charge, or conversely, the depth of discharge (DOD) can be determined by measuring the voltage and/or the specific gravity of the acid with a hydrometer. This will NOT tell you how good (capacity in AH) the battery condition is – only a sustained load test can do that. Voltage on a fully charged battery will read 2.12 to 2.15 volts per cell, or 12.7 volts for a 12 volt battery. At 50% the reading will be 2.03 VPC (Volts Per Cell), and at 0% will be 1.75 VPC or less. Specific gravity will be about 1.265 for a fully charged cell, and 1.13 or less for a totally discharged cell. This can vary with battery types and brands somewhat – when you buy new batteries you should charge them up and let them sit for a while, then take a reference measurement. Many batteries are sealed, and hydrometer reading cannot be taken, so you must rely on voltage. Hydrometer readings may not tell the whole story, as it takes a while for the acid to get mixed up in wet cells. If measured right after charging, you might see 1.27 at the top of the cell, even though it is much less at the bottom. This does not apply to gelled or AGM batteries.
“False” Capacity
A battery can meet all the tests for being at full charge, yet be much lower than it’s original capacity. If plates are damaged, sulfated, or partially gone from long use, the battery may give the appearance of being fully charged, but in reality acts like a battery of much smaller size. This same thing can occur in gelled cells if they are overcharged and gaps or bubbles occur in the gel. What is left of the plates may be fully functional, but with only 20% of the plates left… Batteries usually go bad for other reasons before reaching this point, but it is something to be aware of if your batteries seem to test OK but lack capacity and go dead very quickly under load.
On the table below, you have to be careful that you are not just measuring the surface charge. To properly check the voltages, the battery should sit at rest for a few hours, or you should put a small load on it, such as a small automotive bulb, for a few minutes. The voltages below apply to ALL Lead-acid batteries, except gelled. For gel cells, subtract .2 volts. Note that the voltages when actually charging will be quite different, so do not use these numbers for a battery that is under charge.
Amp-Hour Capacity
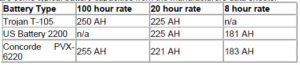
All deep cycle batteries are rated in amp-hours. An amp-hour is one amp for one hour, or 10 amps for 1/10 of an hour and so forth. It is amps x hours. If you have something that pulls 20 amps, and you use it for 20 minutes, then the amp-hours used would be 20 (amps) x .333 (hours), or 6.67 AH. The accepted AH rating time period for batteries used in solar electric and backup power systems (and for nearly all deep cycle batteries) is the “20 hour rate”. This means that it is discharged down to 10.5 volts over a 20 hour period while the total actual amp-hours it supplies is measured. Sometimes ratings at the 6 hour rate and 100 hour rate are also given or comparison and for different applications. The 6-hour rate is often used for industrial batteries, as that is a typical daily duty cycle. Sometimes the 100 hour rate is given just to make the battery look better than it really is, but it is also useful for figuring battery capacity for long-term backup amp-hour requirements.
Why amp-hours are specified at a particular rate:
Because of something called the Peukert Effect. The Peukert value is directly related to the internal resistance of the battery. The higher the internal resistance, the higher the losses while charging and discharging, especially at higher currents. This means that the faster a battery is used (discharged), the LOWER the AH capacity. Conversely, if it is drained slower, the AH capacity is higher. This is important because some folks have chosen to rate their batteries at the 100 hour rate – which makes them look a lot better than they really are.
Here are some typical battery capacities from the manufacturers data sheets:
State of Charge
Here are no-load typical voltages vs state of charge(figured at 10.5 volts = fully discharged, and 77 degrees F). Voltages are for a 12 volt battery system. For 24 volt systems multiply by 2, for 48 volt system, multiply by 4. VPC is the volts per individual cell – if you measure more than a .2 volt difference between each cell, you need to equalize, r your batteries are going bad, or they may be sulfated. These voltages are for batteries that have been at rest for 3 hours or more. Batteries that are being charged will be higher – the voltages while under charge will not tell you anything, you have to let the battery sit for a while. For longest life, batteries should stay in the green zone. Occasional dips into the yellow are not harmful, but continual discharges to those levels will shorten battery life considerably. It is important to realize that voltage measurements are only approximate. The best determination is to measure the specific gravity, but in many batteries this is difficult or impossible. Note the large voltage drop in the last 10%.
10% —> 11.31 —> 1.89
0 —> 10.5 —> 1.75
……………………………………………………………………………………………………..
These voltages are always a bit “iffy”, as they depend on what you consider to be a totally discharged battery. For example, the chart above shows the usual industry standard of 10.5 volts as being 100% discharged. A few charts around though – like for some inverters – will show 100% discharge as being at some other voltage, usually around 11.2 to 11.5.
…………………………………………………………………………………………………….
Battery Charging
Battery charging takes place in 3 basic stages: Bulk, Absorption, and Float.
Bulk Charge – The first stage of 3-stage battery charging. Current is sent to batterie at the maximum safe rate they will accept until voltage rises to near (80-90%) full charge level. Voltages at this stage typically range from 10.5 volts to 15 volts. There is no “correct” voltage for bulk charging, but there may be limits on the maximum current that the battery and/or wiring can take.
Absorption Charge: The 2nd stage of 3-stage battery charging. Voltage remains constant and current gradually tapers off as internal resistance increases during charging. It is during this stage that the charger puts out maximum voltage. Voltages at this stage are typically around 14.2 to 15.5 volts.
Float Charge: The 3rd stage of 3-stage battery charging. After batteries reach full charge, charging voltage is reduced to a lower level (typically 12.8 to 13.2) to reduce gassing and prolong battery life. This is often referred to as a maintenance or trickle charge, since it’s main purpose is to keep an already charged battery from discharging. PWM, or “pulse width modulation” accomplishes the same thing. In PWM, the controller or charger senses tiny voltage drops in the battery and sends very short charging cycles (pulses) to the battery. This may occur several hundred times per minute. It is called “pulse width” because the width of the pulses may vary from a few microseconds to several seconds. Note that for long term float service, such as backup power systems that are seldom discharged, the float voltage should be around 13.02 to 13.20 volts.
Chargers: Most garage and consumer (automotive) type battery chargers are bulk charge only, and have little (if any) voltage regulation. They are fine for a quick boost to low batteries, but not to leave on for long periods. Among the regulated chargers, there are the voltage regulated ones, such as Iota Engineering and Todd, which keep a constant regulated voltage on the batteries. If these are set to the correct voltages for your batteries, they will keep the batteries charged without damage. These are sometimes called “taper charge” – as if that is a selling point. What taper charge really means is that as the battery gets charged up, the voltage goes up, so the amps out of the charger goes down. They charge OK, but a charger rated at 20 amps may only be supplying 5 amps when the batteries are 80% charged. To get around this, certain
chargers have “smart”, or multi-stage controls. These use a variable voltage that starts lower but keeps rising to keep the charging amps much more constant for faster charging.
Battery Charging Voltages and Currents:
Most flooded batteries should be charged at no more than the “C/8” rate for any sustained period. “C/8” is the battery capacity at the 20-hour rate divided by 8. For a 220 AH battery, this would equal 26 Amps. Gelled cells should be charged at no more than the C/20 rate, or 5% of their amp-hour capacity. The Concorde AGM batteries are a special case – the can be charged at up the Cx2 rate, or 200% of the capacity for the bulk charge cycle. However, since very few battery cables can take that much current, we don’t recommend you try this at home. To avoid cable overheating, you should stick to C/4 or less.
Charging at 15.5 volts will give you a 100% charge on Lead-acid batteries. Once the charging voltage reaches 2.583 volts per cell, charging should stop or be reduced to a trickle charge. Note that flooded batteries MUST bubble (gas) somewhat occasionally to insure a full charge, and to mix the electrolyte. Float voltage for Lead-acid batteries should be about 2.15 to 2.23 volts per cell, or about 12.9-13.4 volts for a 12 volt battery. At higher temperatures (over 85 degrees F) this should be reduced to about 2.10 volts per cell.
Never add acid to a battery, except to replace spilled liquid. Distilled or deionized water should be used to top off flooded batteries. Float and charging voltages for gelled batteries are usually about 2/10th volt less than for flooded to reduce water loss. Note that many shunt-type charge controllers sold for solar systems will NOT give you a full charge – check the specifications first. To get a full charge, you must continue to apply a current after the battery voltage reaches the cutoff point of most of these type of controllers. This is why we recommend the charge controls and battery chargers listed in the sections above. Not all shunt type controllers are 100% on or off, but most are.
Flooded battery life can be extended if an equalizing charge is applied every 10 to 40 days. This is a charge that is about 10% higher than normal full charge voltage,
and is applied for about 2 to 16 hours. This makes sure that all the cells are equally charged, and the gas bubbles mix the electrolyte. If the liquid in standard wet cells is not mixed, the electrolyte becomes “stratified”. You can have very strong solution at the top, and very weak at the bottom of the cell. With stratification, you can test a battery with a hydrometer and get readings that are quite a ways off. If you cannot equalize for some reason, you should let the battery sit for at least 24 hours and then use the hydrometer. AGM and gelled should be equalized 2-4 times a year at most (some manufacturers do not recommend an equalizing charge) – check the manufacturers recommendations, especially on gelled.
Battery Aging
As batteries age, their maintenance requirements change. This means longer charging time and/or higher finish rate (higher amperage at the end of the charge). Usually older batteries need to be watered more often. And, their capacity decreases. Internal resistance goes up, so higher voltages may be needed to keep the same rate of charging current.
Mini Factoids
Nearly all batteries will not reach full capacity until cycled 10-30 times. A brand new battery will have a capacity of about 5-10% less than the rated capacity.
Batteries should be watered after charging unless the plates are exposed, then add just enough water to cover the plates. The liquid will expand somewhat when fully charged. After a full charge, the water level should be even in all cells and usually 1/4″ to 1/2″ below the bottom of the fill well in the cell (depends on battery size and type).
In situations where multiple batteries are connected in series, parallel or series/parallel, replacement batteries should be the same size, type and manufacturer (if possible). Age and usage level should be the same as the companion batteries. Do not put a new battery in a pack which is more than 3 months old or has more than 75 cycles. Either replace with all new or use a good used battery. For long life batteries, such as the Surrette and Crown, you can have up to a one year age difference.
The vent caps on flooded batteries should remain on the battery while charging. This prevents a lot of the water loss and splashing that may occur when they are bubbling. The only exception we know of is that Hydrocaps should be removed when equalizing.
When you first buy a new set of flooded (wet) batteries, you should fully charge and equalize them, and then take a hydrometer reading for future reference. Since not all batteries have exactly the same acid strength, this will give you a baseline for future readings. When using a small solar panel to keep a float (maintenance) charge on a battery (without using a charge controller), choose a panel that will give a maximum output of about 1/300th to 1/1000th of the amp-hour capacity. For a pair of 200 Amp-Hour golf cart batteries, that would be about a 1 to 5 watt panel – the smaller panel if you get 5 or more hours of sun per day, the larger one for those long cloudy winter days in the Northeast.
Lead-acid batteries do NOT have a memory, and the rumor that they should be fully discharged to avoid this “memory” is totally false and will lead to early battery failure.
Inactivity can be extremely harmful to a battery, especially if not 100% charged. It is a VERY poor idea to buy new batteries and “save” them for later. Either buy them when you need them, or keep them on a continual trickle charge. The best thing – if you buy them, use them.
Only clean water should be used for cleaning the outside of batteries. Solvents or spray cleaners should not be used. Contrary to what you may have heard, it is usually not a good idea to use baking soda to neutralize any acid on the outside of a battery. Even a tiny amount getting inside will also neutralize acid IN the battery. If you need to use it, make sure all the caps are on the battery. Mixing up a tablespoon or so in a small glass of water works for neutralizing any acid around the posts – but again, be careful not to get any inside.
Who is Peukert, and why should I care?
The true capacity of a battery depends on the rate of discharge. The faster the rate of discharge, the less total amp-hours that can be delivered. This was first described back in 1897 by a researcher named Peukert. The Peukert’s number essentially tells you the effective internal resistance of the battery. Peukert applies to ALL batteries of any type (except some high end Lithium-Ion), not just the Lead-acid normally used.
A Peukert value of 1 would be the ideal battery, which does not exist. The higher the number, the more internal resistance – which translates into more losses and heat. Typical batteries currently sold range from around 1.07 up to as high as 1.6. Typical for most flooded is around 1.2, for deep cycle AGM around 1.1. Anything over about 1.3 is pretty bad, and indicates a junk battery, or one that is pretty old and/or sulfated.
The “Peukert Capacity” of a battery is the total number of amp-hours that you can get from a battery when discharged steadily at exactly one amp – or basically just how many hours it takes for the battery to drop to 10.5 volts at 1 amp draw.
Some Peukert Exponent values (not complete, just for info).
- Trojan T-105 = 1.25
- Concorde AGM = 1.06
- Optima 750S = 1.109
- US Battery 2200 = 1.20
- Hawker Genesis = 1.11
More information – Manufacturers Websites
- www.jgdarden.com/batteryfaq/carfaq7.htm – another very good site for lots of battery
info. - www.usbattery.com – some good information and data.
- www.trojanbattery.com – not a lot of real technical info here, but has all the
specifications. - www.surrette.com – Specs and data on the Surrette deep cycle and marine batteries
- www.concordebattery.com – specs and data on all Concorde batteries.
~ Submitted by Mike S., 2017